Einhorn reflects on milestone anniversaries

As Hoosier Cancer Research Network celebrates our 30th anniversary, we acknowledge another significant milestone: the 40th anniversary of the discovery of the cure for testicular cancer by HCRN co-founder and board member, Lawrence H. Einhorn, MD. Dr. Einhorn joined the Indiana University School of Medicine in 1973, just one year before his groundbreaking discovery testing […]
Caring comes naturally for Turner Award winner Pierce

From an early age, Gladys Pierce, RN, OCN, knew she was destined for a career in nursing. She wanted to help people, and volunteered at a nursing home during high school. More than 40 years later, Pierce remains as committed as ever to providing care in her role as nurse coordinator for the Oncology Research […]
Ladies Auxiliary to the VFW donates $12,500 to HCRN

The Indiana Department of the Ladies Auxiliary to the Veterans of Foreign Wars recently donated nearly $12,500 to Hoosier Cancer Research Network (formerly known as Hoosier Oncology Group). The generous gift is just one example of the Ladies Auxiliary’s long-standing support for cancer research. For 100 years, the Ladies Auxiliary to the VFW has served […]
Galsky receives Danny Danielson Translational Innovation Award

Hoosier Cancer Research Network, formerly known as Hoosier Oncology Group, recently honored Matthew Galsky, MD, as recipient of the Danny Danielson Translational Innovation Award. The $10,000 award, granted by the Walther Cancer Foundation through the generous support of business and civic leader Donald C. “Danny” Danielson, is given twice each year to investigators working in […]
Matei receives Fisher Young Investigator Award
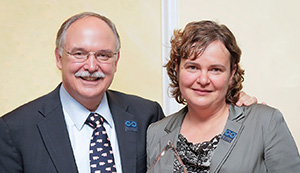
Hoosier Cancer Research Network, formerly known as Hoosier Oncology Group, recently honored Daniela Matei, MD, as the 2014 recipient of the George and Sarah Jane Fisher Young Investigator Award. Dr. Matei received her medical degree in her native Romania before moving to the United States and completed her residency at SUNY at Stony Brook, NY, […]
Matei receives 2011 Research Frontiers Trailblazer Award
Dr. Daniela Matei received the Research Frontiers Trailblazer Award for her discoveries related to the biology of ovarian cancer at the second annual Research Day conference. The Trailblazer award honors outstanding IUPUI researchers who are “showing great promise in becoming nationally and internationally known for their accomplishments in advancing the frontiers of knowledge.”
Danielson receives Terry Hoeppner Patient Advocacy Award

Hoosier Cancer Research Network, formerly known as Hoosier Oncology Group, recently honored Donald C. “Danny” Danielson with the 2013 Terry Hoeppner Patient Advocacy Award in recognition of his long-standing support for cancer research. The award, named in memory of beloved Indiana University football coach Terry Hoeppner, who died from brain cancer in 2007, honors individuals […]
Courage to Climb concert set for August 16

Columbus North High School in Columbus, Ind., will host the 2014 Courage to Climb concert on Saturday, Aug. 16, at 7:00 pm in Erne Auditorium. The annual concert honors Columbus North choir director and cancer survivor Janie Gordon. “More than 40 community members will share their talents and their stories of personal experiences with cancer,” […]
HCRN honors five during 30th anniversary dinner
Hoosier Cancer Research Network (HCRN) recently marked its 30th anniversary with a dinner and presentation of awards. The event featured comments by HCRN co-founder Patrick J. Loehrer, Sr., MD and chairman of the board Christopher A. Fausel, PharmD, MHA, BCOP. HCRN honored five individuals during the June 19 event in Indianapolis.
HCRN honors five during 30th anniversary dinner
Hoosier Cancer Research Network (HCRN) recently marked its 30th anniversary with a dinner and presentation of awards. The event featured comments by HCRN co-founder Patrick J. Loehrer, Sr., MD and chairman of the board Christopher A. Fausel, PharmD, MHA, BCOP. HCRN honored five individuals during the June 19 event in Indianapolis.
HCRN annual report highlights growth and transition

Hoosier Cancer Research Network, formerly known as Hoosier Oncology Group, has released its 2013 annual report. The report features articles highlighting the transition in the Chief Scientific Officer position from Noah Hahn, MD, to Bert H. O’Neil, MD, as well as profiles of donors and award winners. The report also includes financial statements and acknowledgement […]
HCRN annual report highlights growth and transition

Hoosier Cancer Research Network, formerly known as Hoosier Oncology Group, has released its 2013 annual report. The report features articles highlighting the transition in the Chief Scientific Officer position from Noah Hahn, MD, to Bert H. O’Neil, MD, as well as profiles of donors and award winners. The report also includes financial statements and acknowledgement […]
Six HCRN studies featured at ASCO 2014
Hoosier Cancer Research Network (HCRN), formerly known as Hoosier Oncology Group, joined nearly 30,000 of the world’s leading cancer experts in Chicago recently for the Annual Meeting of the American Society of Clinical Oncology (ASCO). Six HCRN studies were featured in oral abstract, poster highlights, and general poster sessions during the May 30-June 3 event. […]
Six HCRN studies featured at ASCO 2014
Hoosier Cancer Research Network (HCRN), formerly known as Hoosier Oncology Group, joined nearly 30,000 of the world’s leading cancer experts in Chicago recently for the Annual Meeting of the American Society of Clinical Oncology (ASCO). Six HCRN studies were featured in oral abstract, poster highlights, and general poster sessions during the May 30-June 3 event. […]
Robin T. Zon, MD, FACP, FASCO, to present Education Session at ASCO

Former Hoosier Cancer Research Network vice-chair Robin T. Zon, MD, FACP, FASCO, will chair an Education Session during the American Society of Clinical Oncology (ASCO) annual meeting in Chicago. Zon, along with Jeffrey S. Abrams, MD, of the National Cancer Institute at the National Institutes of Health, and Nicholas J. Robert, MD, of Virginia Cancer […]
Robin T. Zon, MD, FACP, FASCO, to present Education Session at ASCO

Former Hoosier Cancer Research Network vice-chair Robin T. Zon, MD, FACP, FASCO, will chair an Education Session during the American Society of Clinical Oncology (ASCO) annual meeting in Chicago. Zon, along with Jeffrey S. Abrams, MD, of the National Cancer Institute at the National Institutes of Health, and Nicholas J. Robert, MD, of Virginia Cancer […]
New breast trial incorporates advanced genomic technology

Indiana University cancer researchers are testing whether therapy incorporating advanced genomic technology will provide better outcomes than current treatments for those with an aggressive form of breast cancer. Researchers at the Indiana University Melvin and Bren Simon Cancer Center, led by Bryan Schneider, M.D., associate professor of medicine, and Milan Radovich, Ph.D., assistant professor of […]
New breast trial incorporates advanced genomic technology

Indiana University cancer researchers are testing whether therapy incorporating advanced genomic technology will provide better outcomes than current treatments for those with an aggressive form of breast cancer. Researchers at the Indiana University Melvin and Bren Simon Cancer Center, led by Bryan Schneider, M.D., associate professor of medicine, and Milan Radovich, Ph.D., assistant professor of […]
Hoosier Oncology Group announces new name and website

Hoosier Oncology Group, an independent nonprofit cancer research organization in Indianapolis announces it is changing its name to Hoosier Cancer Research Network. The change, which goes into effect May 15, reflects the company’s steady growth over 30 years into a nationwide network of more than 130 member sites and more than 400 physicians and research […]
Hoosier Oncology Group announces new name and website

Hoosier Oncology Group, an independent nonprofit cancer research organization in Indianapolis announces it is changing its name to Hoosier Cancer Research Network. The change, which goes into effect May 15, reflects the company’s steady growth over 30 years into a nationwide network of more than 130 member sites and more than 400 physicians and research […]
Hoosier Cancer Research Network co-founder elected AACR fellow

Lawrence Einhorn, M.D., the physician-researcher who developed the cure for testicular cancer, has been elected a fellow of the American Association for Cancer Research. Dr. Einhorn, Indiana University Distinguished Professor and a physician-researcher at the Indiana University Melvin and Bren Simon Cancer Center, will be inducted into the American Association for Cancer Research Academy Class […]
Hoosier Cancer Research Network co-founder elected AACR fellow

Lawrence Einhorn, M.D., the physician-researcher who developed the cure for testicular cancer, has been elected a fellow of the American Association for Cancer Research. Dr. Einhorn, Indiana University Distinguished Professor and a physician-researcher at the Indiana University Melvin and Bren Simon Cancer Center, will be inducted into the American Association for Cancer Research Academy Class […]
Hahn to lead bladder cancer program at Johns Hopkins
Noah Hahn, MD, recently concluded his tenure as chief scientific officer of the Hoosier Oncology Group and associate professor of medicine at the Indiana University Melvin and Bren Simon Cancer Center (IUSCC). Hahn joins the Johns Hopkins School of Medicine and the Sidney Kimmel Comprehensive Cancer Center as bladder cancer program director starting April 2014.
Hahn to lead bladder cancer program at Johns Hopkins
Noah Hahn, MD, recently concluded his tenure as chief scientific officer of the Hoosier Oncology Group and associate professor of medicine at the Indiana University Melvin and Bren Simon Cancer Center (IUSCC). Hahn joins the Johns Hopkins School of Medicine and the Sidney Kimmel Comprehensive Cancer Center as bladder cancer program director starting April 2014.
Shahda receives inaugural Danny Danielson Translational Innovation Award

Hoosier Oncology Group recently honored Safi Shahda, M.D., as the inaugural recipient of the Danny Danielson Translational Innovation Award. The $10,000 award, granted by the Walther Cancer Foundation through the generous support of Danny Danielson, is given twice each year to an investigator to support the correlative components of clinical trial protocols when financial support […]
Shahda receives inaugural Danny Danielson Translational Innovation Award

Hoosier Oncology Group recently honored Safi Shahda, M.D., as the inaugural recipient of the Danny Danielson Translational Innovation Award. The $10,000 award, granted by the Walther Cancer Foundation through the generous support of Danny Danielson, is given twice each year to an investigator to support the correlative components of clinical trial protocols when financial support […]
Jalal receives Fisher Young Investigator Award

Hoosier Oncology Group recently honored Shadia Jalal, M.D., as the 2013 recipient of the George and Sarah Jane Fisher Young Investigator Award. The $10,000 award, established in 2011 by Dr. William B. Fisher through the George and Sarah Jane Fisher Fund, is given annually to an Indiana University oncology fellow or faculty member who has […]
Jalal receives Fisher Young Investigator Award

Hoosier Oncology Group recently honored Shadia Jalal, M.D., as the 2013 recipient of the George and Sarah Jane Fisher Young Investigator Award. The $10,000 award, established in 2011 by Dr. William B. Fisher through the George and Sarah Jane Fisher Fund, is given annually to an Indiana University oncology fellow or faculty member who has […]
Hoosier Cancer Research Network receives $1.9 million gift for cancer research
Hoosier Oncology Group in Indianapolis has received a $1.9 million gift from the estate of Margaret M. Weeks of Evansville, Ind. Hoosier Oncology Group will use the gift to further cancer research through the conduct of clinical trials. The gift is one of the largest donations in the organization’s history.
Hoosier Cancer Research Network receives $1.9 million gift for cancer research
Hoosier Oncology Group in Indianapolis has received a $1.9 million gift from the estate of Margaret M. Weeks of Evansville, Ind. Hoosier Oncology Group will use the gift to further cancer research through the conduct of clinical trials. The gift is one of the largest donations in the organization’s history.
GU02-41 prostate cancer study published in Clinical Genitourinary Cancer
Congratulations to the GU02-41 team!
GU02-41 prostate cancer study published in Clinical Genitourinary Cancer
Congratulations to the GU02-41 team!
Hoosier Cancer Research Network Welcomes New Chairman
Hoosier Oncology Group is very pleased to announce that Christopher A. Fausel, PharmD, MHA, BCOP, was appointed Chairman of the Board of Directors on July 31, 2013. Dr. Fausel is the Clinical Manager of Oncology Pharmacy at the Indiana University Simon Cancer Center (IUSCC) in Indianapolis.
Hoosier Cancer Research Network Welcomes New Chairman
Hoosier Oncology Group is very pleased to announce that Christopher A. Fausel, PharmD, MHA, BCOP, was appointed Chairman of the Board of Directors on July 31, 2013. Dr. Fausel is the Clinical Manager of Oncology Pharmacy at the Indiana University Simon Cancer Center (IUSCC) in Indianapolis.
Cancer Research Contributors Recognized by Indiana General Assembly
The Hoosier Oncology Group is proud to be among the major contributors acknowledged in House Concurrent Resolution No. 44 of the State of Indiana General Assembly, honoring and recognizing the contributions of those Hoosiers who have helped fight the War on Cancer as cancer researchers or as patients serving as research participants. This recognition is […]
Cancer Research Contributors Recognized by Indiana General Assembly
The Hoosier Oncology Group is proud to be among the major contributors acknowledged in House Concurrent Resolution No. 44 of the State of Indiana General Assembly, honoring and recognizing the contributions of those Hoosiers who have helped fight the War on Cancer as cancer researchers or as patients serving as research participants. This recognition is […]
Hoosier Cancer Research Network Researcher to be Featured in Discovery Channel documentary
IU Simon Cancer Center breast cancer oncologist George Sledge, MD, is featured in the Discovery Channel documentary “Getting Personal: The Shifting Landscape of Cancer Care.” New research is shedding light on the genetic causes of cancer, as well as the molecular make-up of individual cancers, paving the path for more effective therapies and more personalized […]
Hoosier Cancer Research Network Researcher to be Featured in Discovery Channel documentary
IU Simon Cancer Center breast cancer oncologist George Sledge, MD, is featured in the Discovery Channel documentary “Getting Personal: The Shifting Landscape of Cancer Care.” New research is shedding light on the genetic causes of cancer, as well as the molecular make-up of individual cancers, paving the path for more effective therapies and more personalized […]
Matei receives 2011 Research Frontiers Trailblazer Award
Dr. Daniela Matei received the Research Frontiers Trailblazer Award for her discoveries related to the biology of ovarian cancer at the second annual Research Day conference. The Trailblazer award honors outstanding IUPUI researchers who are “showing great promise in becoming nationally and internationally known for their accomplishments in advancing the frontiers of knowledge.”
LUN05-99 manuscript accepted by the Journal of Thoracic Oncology
Hoosier Oncology is pleased to announce that study LUN05-99 – “Paclitaxel plus Bevacizumab in Patients with Chemosensitive Relapsed Small Cell Lung Cancer (SCLC): A Safety, Feasibility and Efficacy Study” has been accepted by the Journal of Thoracic Oncology. Congratulations to Dr. Jalal and the entire team.
LUN05-99 manuscript accepted by the Journal of Thoracic Oncology
Hoosier Oncology is pleased to announce that study LUN05-99 – “Paclitaxel plus Bevacizumab in Patients with Chemosensitive Relapsed Small Cell Lung Cancer (SCLC): A Safety, Feasibility and Efficacy Study” has been accepted by the Journal of Thoracic Oncology. Congratulations to Dr. Jalal and the entire team.
LUN06-116 published in Journal of Thoracic Oncology
We are pleased to announce that LUN-116 abstract has been published in the November 2010 issue of the Journal of Thoracic Oncology. Congratulations to Dr. Hanna and team! Click here to review the abstract online.
LUN06-116 published in Journal of Thoracic Oncology
We are pleased to announce that LUN-116 abstract has been published in the November 2010 issue of the Journal of Thoracic Oncology. Congratulations to Dr. Hanna and team! Click here to review the abstract online.
ASCO president and Hoosier Oncology Group investigator Dr. George Sledge testifies before president’s cancer panel

ASCO President, Dr. George W. Sledge, was among the clinician, government and industry leaders selected to testify before the President’s Cancer Panel in Boston on September 22, 2010. The meeting, titled, “The Future of Cancer Research: Accelerating Scientific Innovation,” focused on assessing the past 40 years of cancer research and exploring potential future enhancements and […]
ASCO president and Hoosier Oncology Group investigator Dr. George Sledge testifies before president’s cancer panel

ASCO President, Dr. George W. Sledge, was among the clinician, government and industry leaders selected to testify before the President’s Cancer Panel in Boston on September 22, 2010. The meeting, titled, “The Future of Cancer Research: Accelerating Scientific Innovation,” focused on assessing the past 40 years of cancer research and exploring potential future enhancements and […]
Longtime Hoosier Oncology Group investigators hold national leadership roles

George Sledge, M.D., can add yet one more title to his long list of impressive credentials and titles: President of the American Society of Clinical Oncology (ASCO). Dr. Sledge is most known for his ground-breaking work in breast cancer research, with a specific interest in the use of angiogenesis blockers, or disruption of the blood […]
Longtime Hoosier Oncology Group investigators hold national leadership roles

George Sledge, M.D., can add yet one more title to his long list of impressive credentials and titles: President of the American Society of Clinical Oncology (ASCO). Dr. Sledge is most known for his ground-breaking work in breast cancer research, with a specific interest in the use of angiogenesis blockers, or disruption of the blood […]