Investigator Spotlight: Joseph Chao, MD

This month, Hoosier Cancer Research Network (HCRN) features our member City of Hope and Joseph Chao, MD, a medical oncologist and researcher at the cancer center. Dr. Chao is a member of the HCRN Gastrointestinal Clinical Trial Working Group and has participated as an investigator on the HCRN GI16-288 and GI17-319 studies. Research Interests and […]
Study finds presence of ctDNA and CTCs after neoadjuvant chemo in triple-negative breast cancer is associated with disease recurrence

Results from a multi-site clinical trial for patients with triple-negative breast cancer (TNBC) show that genomic testing for circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs) after neoadjuvant chemotherapy can be used to detect residual disease and identify which patients could be at high risk of relapse. The results were recently published in JAMA […]
Investigator Spotlight: Nasser Hanna, MD

This month, Hoosier Cancer Research Network (HCRN) features our member Indiana University Melvin and Bren Simon Comprehensive Cancer Center and Nasser Hanna, MD, a medical oncologist and researcher at the cancer center. Dr. Hanna is a former chairman of the HCRN board of directors and a current member of the HCRN Thoracic Clinical Trial Working […]
Investigator Spotlight: Arkadiusz Z. Dudek, MD, PhD, FACP

This month, Hoosier Cancer Research Network (HCRN) features our member HealthPartners Institute and Arkadiusz Z. Dudek, MD. He’s a medical oncologist at Regions Hospital Cancer Care Center in St. Paul, Minn., an investigator at HealthPartners Institute, and a professor in the Department of Medicine at University of Minnesota. Research Interests and Expertise Dr. Dudek is […]
Researchers study combination immunotherapy treatment for ocular melanoma

A Hoosier Cancer Research Network study for patients with high-risk ocular melanoma, a rare type of cancer affecting the eye, is now open for accrual. According to the National Cancer Institute, ocular melanoma often doesn’t present early signs or symptoms. Typically, patients with ocular melanoma can choose to have surgery to remove the eye, called […]
Investigator Spotlight: Daniel Vaena, MD

This month, Hoosier Cancer Research Network (HCRN) features our member West Cancer Center in Memphis, Tenn., and Daniel Vaena, MD, a medical oncologist and hematologist and director of the Genitourinary and Phase I programs at West Cancer Center and Research Institute. Research Interests and Expertise Dr. Vaena has particular interests in novel immunotherapy and molecular […]
HCRN studies selected for ASCO20 oral abstract, posters

The American Society of Clinical Oncology’s ASCO20 Virtual Scientific Program will feature abstracts from three Hoosier Cancer Research Network studies. The program, taking place May 29-31, will feature more than 250 oral abstract presentations and 2,500 poster presentations from 24 disease-based and specialty tracks. The abstracts featuring HCRN studies include an oral abstract for GU16-260, […]
Study tests immunotherapy and selective bladder sparing for patients with muscle-invasive bladder cancer

A Hoosier Cancer Research Network study for adult patients with muscle-invasive bladder cancer (MIBC) will help doctors determine whether some patients could forego bladder removal and receive standard chemotherapy drugs and the immunotherapy drug, nivolumab. It will also test whether adding nivolumab to chemotherapy drugs, gemcitabine and cisplatin, works better than chemotherapy alone for treating […]
Investigator Spotlight: Ryan Gentzler, MD

This month, Hoosier Cancer Research Network (HCRN) features our member University of Virginia Cancer Center and Ryan Gentzler, MD, MS, a thoracic medical oncologist and clinical investigator at the UVA Emily Couric Clinical Cancer Center. Research Interests and Expertise Much of Dr. Gentzler’s research focuses on developing new drugs and therapeutic strategies for the treatment […]
HCRN supporters contribute more than $5,400 through Reps for Research

Supporters of Hoosier Cancer Research Network (HCRN) donated more than $5,400 this spring through the Reps for Research challenge. The annual event, led by HCRN Chairman Christopher A. Fausel, PharmD, and Jeanne Schilder, MD, involves contributions tied to the number of bench press repetitions they complete during the Arnold Sports Festival 5K Pump and Run […]
HCRN investigators report switch maintenance pembrolizumab leads to additional objective responses, prolongs progression-free survival in some patients with metastatic urothelial cancer

In a phase II study reported in the Journal of Clinical Oncology, Hoosier Cancer Research Network (HCRN) investigators report that switch maintenance pembrolizumab leads to additional objective responses and significantly prolongs progression-free survival in patients with metastatic urothelial cancer achieving at least stable disease with first-line platinum-based chemotherapy. The multi-center investigator-initiated study, “A Randomized, Double-Blinded, […]
A Letter from our Chairman: 2019 Annual Report

As the books closed on 2019, Hoosier Cancer Research Network approached a familiar juncture. The organization’s steady growth, both in numbers as well as in the range and quality of service we provide, have led us to full capacity in our present location. There is only so much creative reorganizing you can do in a […]
A letter from our CSO: 2019 Annual Report

As a member of the Hoosier Cancer Research Network, I am grateful to collaborate with outstanding investigators in the development of multi-center, investigator-initiated clinical trials. I’ve seen the value of this collaboration throughout my own professional development: from my oncology fellowship under the mentorship of Dr. Larry Einhorn and Dr. Nasser Hanna to my current […]
Rangaraju values Fisher Award support for leukemia research

Since pathology class in medical school in Nepal, Sravanti Rangaraju, MBBS, this year’s George and Sarah Jane Fisher Young Investigator Award recipient, has found studying peripheral blood smears intriguing. “When you look at blood cells under a microscope, you can get clues to several diagnoses just based on that,” said Dr. Rangaraju, a third-year hematology […]
Investigator Spotlight: Peter H. O’Donnell, MD

This month, Hoosier Cancer Research Network (HCRN) features our member The University of Chicago Medicine Comprehensive Cancer Center, and Peter H. O’Donnell, MD, deputy director, Center for Personalized Therapeutics; associate director, Clinical Pharmacology and Pharmacogenomics Fellowship Program; and associate director, Paul Calabresi Oncology Training Program (K12) at The University of Chicago.
We are moving. Here’s what you need to know.
Hoosier Cancer Research Network is moving to a new office location this spring, and our mailing address is changing. The following FAQ outlines how this change will impact certain areas of our operations. If you have any questions not covered below, please call our offices at 317-921-2050, or send an email to contact@hoosiercancer.org.
Multi-site phase II study tests PARP inhibitor olaparib in metastatic urothelial cancer patients with somatic DDR alterations

A Hoosier Cancer Research Network multi-site phase II study led by Icahn School of Medicine at Mount Sinai/Tisch Cancer Institute is testing the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib in metastatic urothelial cancer patients with somatic DNA damage response (DDR) alterations in their cells. The study is open to accrual at HCRN member sites, including: […]
Investigator Spotlight: Guru P. Sonpavde, MD

This month, Hoosier Cancer Research Network (HCRN) features our member Dana-Farber Cancer Institute, and the Institute’s Guru P. Sonpavde, MD, a long-time HCRN investigator.
Study tests atezolizumab plus chemotherapy in advanced urothelial cancer after progression on PD-1 or PD-L1 therapy

Indiana University Melvin and Bren Simon Cancer Center is leading a phase II clinical trial that will test the PD-L1 checkpoint inhibitor atezolizumab plus chemotherapy in patients with advanced urothelial cancer who are cisplatin-ineligible and whose cancer worsened after treatment with a PD-1 or PD-L1 inhibitor. The study, HCRN-GU17-295, is open to accrual at the […]
Investigators present GU17-295 poster at GU ASCO

Hoosier Cancer Research Network investigators presented a trials in progress poster featuring the HCRN study GU17-295 during the American Society of Clinical Oncology’s 2020 Genitourinary Cancers Symposium on Friday, Feb. 14, in San Francisco, Calif. The poster, titled, “Phase II trial of atezolizumab plus chemotherapy after progression on single-agent PD-1 or PD-L1 inhibitor in cisplatin […]
Investigator Spotlight: Autumn McRee, MD

This month, Hoosier Cancer Research Network (HCRN) highlights our member the University of North Carolina Lineberger Comprehensive Cancer Center. Autumn McRee, MD, associate professor of medicine and director of the GI Clinical Trials Program at the cancer center, shares her research interests in this investigator spotlight.
Investigator Spotlight: Maitri Kalra, MD

This month, Hoosier Cancer Research Network (HCRN) highlights the IU Health Ball Memorial Cancer Center as a featured member of our network. Maitri Kalra, MD, a medical oncologist at the cancer center, shares her research interests in this spotlight.
HCRN esophageal cancer study presented as trial in progress at GI ASCO

The Hoosier Cancer Research Network study, HCRN ESO17-325, a phase II study for adults with previously treated metastatic esophageal cancer, with one of three genetic mutations, including homologous recombination (HR) in tumor tissue; defective or loss of heterozygosity (LOH) in tumor tissue; or a germline mutation in the blood, was featured as a trials in […]
Investigator Spotlight: Jue Wang, MD, FACP

This month, Hoosier Cancer Research Network highlights the work of University of Arizona Cancer Center at Dignity Health St. Joseph’s as a featured member of Hoosier Cancer Research Network. Jue Wang, MD, FACP, professor of medicine and interdisciplinary oncology and section leader of the Genitourinary Oncology Division at the University of Arizona, shares his research […]
IU School of Medicine researchers predict which triple negative breast cancer patients may avoid recurrence and which are at high-risk of relapse

Indiana University School of Medicine researchers have discovered how to predict whether triple negative breast cancer will recur, and which women are likely to remain disease-free. They presented their findings on December 13, 2019, at the San Antonio Breast Cancer Symposium, the most influential gathering of breast cancer researchers and physicians in the world. Milan […]
HCRN study highlighted at international melanoma meeting
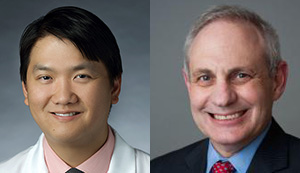
Hoosier Cancer Research Network investigators highlighted an HCRN melanoma study as a poster presentation during the Society for Melanoma Research 16th International Congress, Nov. 20-23, 2019, in Salt Lake City, Utah. The authors, led by sponsor-investigator Suthee Rapisuwon, MD (pictured left), and co-investigator Michael B. Atkins, MD, both of Georgetown University Lombardi Comprehensive Cancer Center, […]
Investigator Spotlight: Rachel E. Sanborn, MD

This month, Hoosier Cancer Research Network highlights the Providence Cancer Institute in Portland, Ore., as a featured member of our network. Rachel E. Sanborn, MD, is a medical oncologist at Portland Cancer Institute and is a member and co-chair of the HCRN Thoracic Clinical Trial Working Group.
Study tests entinostat in combination with ipilimumab and nivolumab in metastatic RCC

A single arm, phase II clinical trial for patients with metastatic renal cell carcinoma (RCC), the most common type of kidney cancer, is testing the histone deacetylase (HDAC) inhibitor entinostat in combination with ipilimumab and nivolumab in patients previously treated with nivolumab and ipilimumab or nivolumab alone. The study is open to accrual at Indiana […]
Investigator Spotlight: Anup Kasi, MD, MPH

This month, Hoosier Cancer Research Network highlights the University of Kansas Medical Center as a featured member of our network. Anup Kasi, MD, MPH, assistant professor of oncology at the medical center and a member of the HCRN Gastrointestinal Clinical Trial Working Group, shares his research interests and educational background in this investigator spotlight.
HCRN studies presented at 2019 World Conference on Lung Cancer

Investigators reported results from two HCRN studies during poster sessions at the 2019 World Conference on Lung Cancer, September 7-9 in Barcelona, Spain. Study authors, led by Greg Durm, MD (pictured left), of the Indiana University Melvin and Bren Simon Cancer Center, reported results of the LUN14-179 study, in a poster titled “ChemoXRT w/ Consolidation […]
Study compares high-dose IL-2 and HDAC inhibitor entinostat to IL-2 alone

A phase II, open label study for patients with renal cell carcinoma (RCC), the most common type of kidney cancer, is currently enrolling subjects at selected Hoosier Cancer Research Network sites. The HCRN GU17-289 study, led by researchers at the Indiana University Melvin and Bren Simon Cancer Center in Indianapolis, Ind., compares the positive and […]
Jalal directs research priorities for HCRN as new chief scientific officer

Shadia Jalal, MD, chief scientific officer for Hoosier Cancer Research Network and a thoracic medical oncologist and researcher at Indiana University Melvin and Bren Simon Cancer Center, understands the importance of process in directing research priorities and getting studies off the ground in a timely fashion. Since succeeding Bert O’Neil, MD, as CSO in April […]
Study tests atezolizumab with bevacizumab in advanced bladder cancer

Each year in the United States, more than 70,000 patients are diagnosed with bladder cancer, and more than 14,000 will die from their disease. The current standard for treating bladder cancer involves chemotherapy, but this approach is not adequate for many patients, particularly those whose disease has metastasized, or spread to other parts of the […]
ASCO selects HCRN studies for oral abstract and poster sessions

Two Hoosier Cancer Research Network studies, GU14-182 and MEL17-309, will be presented during the 2019 American Society of Clinical Oncology (ASCO) Annual Meeting, May 31 – June 4, in Chicago. HCRN GU14-182, a randomized, double-blinded, phase II study of maintenance pembrolizumab versus placebo after first-line chemotherapy in patients with metastatic urothelial cancer, will be featured […]
GU14-188 abstract presented at AUA 2019 annual meeting

The Hoosier Cancer Research Network study HCRN GU14-188, a multicenter Phase Ib/II study of neoadjuvant pembrolizumab and cisplatin chemotherapy for muscle-invasive urothelial cancer, was featured as a podium session abstract May 5, 2019, during the American Urological Association (AUA) Annual Meeting in Chicago. The multi-center study is led by sponsor-investigator Christopher Hoimes, DO (pictured), of […]
HCRN announces ASCO 2019 meetings

Hoosier Cancer Research Network will host meetings for Clinical Trial Working Groups during ASCO 2019. The following meetings will be at the Holiday Inn Chicago Mart Plaza River North, 350 West Mart Center Drive, Chicago, Ill. See map. All times are CT. Refreshments will be served at all meetings.
Reps for Research contributors donate more than $6,000 to HCRN

For the fifth consecutive year, supporters of Hoosier Cancer Research Network (HCRN) donated thousands of dollars this spring through the Reps for Research challenge. The annual event, led this year by (pictured, from left) HCRN Chairman Christopher A. Fausel, PharmD; gynecologic oncologist Jeanne Schilder, MD; and nurse practitioner Courtney Cortez, involves donations tied to the […]
O’Neil concludes tenure as HCRN chief scientific officer

After five years as chief scientific officer for Hoosier Cancer Research Network (HCRN), Bert H. O’Neil, MD, recently stepped down from the role, a move that coincided with his transition from Indiana University to Eli Lilly and Company. Dr. O’Neil continues to serve as a member of the HCRN Board of Directors.
GI14-198 study presented at GI ASCO 2019

The Hoosier Cancer Research Network study HCRN GI14-198, a Phase II randomized, double-blind, study of mfolfirinox plus ramucirumab versus mfolfirinox plus placebo in advanced pancreatic cancer patients, was featured as a trials in progress poster during the 2019 Gastrointestinal Cancers Symposium, Jan. 18 in San Francisco, CA. The multi-center study is led by sponsor-investigator Walid […]
A message from our chairman

HCRN Annual Report 2018 For the majority of its 35-year history, Hoosier Cancer Research Network (HCRN) has been one of the best kept secrets in clinical oncology research. Through steady leadership and a responsive organizational structure, HCRN has quietly navigated decades of change in the field of oncology, always keeping the needs of our members […]
A message from our chief scientific officer

HCRN Annual Report 2018 Hoosier cancer Research Network enjoys a unique synergy with our research collaborators that few others can claim. An Indiana-based nonprofit organization with a nationwide impact, HCRN operates at a crossroads where the interests of academic institutions, health networks, and industry funders merge to create a shared avenue for the development, management, […]
HCRN honors UNC’s Anthony Drier for excellence in clinical research

Anthony Drier, clinical research coordinator at the University of North Carolina at Chapel Hill recently received the 2018 Sandra Turner Excellence in Clinical Research Award for his effort in coordinating cancer research studies with great attention to detail, service, and timeliness. The Sandra Turner Excellence in Clinical Research Award is awarded annually by the Hoosier […]
Fisher award recipient Shahid Ahmed credits mentoring as key to professional success

Having good mentors is something that this year’s George and Sarah Jane Fisher Young Investigator Award recipient, Shahid Ahmed, MD, values greatly. Through his personal drive and strong mentors, Dr. Ahmed pursued learning opportunities and research that led him to Indiana University School of Medicine, where he is currently exploring novel oncology research as a […]
Mesothelioma study tests combination of antiangiogenic therapy and immunotherapy

A single arm phase II study, LUN15-299, for patients who have been previously treated for mesothelioma is being conducted by the Hoosier Cancer Research Network and led by HealthPartners Regions Cancer Care in St. Paul, Minn. The clinical trial will test how the addition of an antiangiogenic therapy drug, ramucirumab, will work with nivolumab, an […]
Peprah honored for excellence and compassion in clinical care coordination

Ensuring the timely execution of multiple clinical trials at a major university is no small task. Nana Peprah, MPH, clinical research coordinator at the University of Chicago, may see herself as only a coordinator, but her efforts in keeping clinical research trials on schedule through compassion and service to cancer patients and clinical staff have […]
ADAPT-BLADDER study compares novel immunotherapy combinations and BCG re-treatment in non-muscle invasive bladder cancer

Hoosier Cancer Research Network (HCRN) has opened a study for patients with non-muscle invasive bladder cancer who were previously treated with the Bacillus Calmette-Guerin (BCG) vaccine. The randomized phase I/II clinical trial will test standard re-treatment with BCG against the immunotherapeutic agent durvalumab combined with BCG, or durvalumab combined with radiation therapy.
Patient advocate offers a sanctuary for loved ones to gather and rest

Families who fight cancer along with their ailing loved one grapple with many issues, from health decisions and a myriad of emotions to logistical and financial concerns. The range of concerns is something Becky Armbruster, this year’s Terry Hoeppner Patient Advocacy Award winner, recalls very poignantly. From the time her late husband, Doug, underwent treatment […]
HCRN recognizes four individuals with 2018 awards

Hoosier Cancer Research Network honored four individuals for their outstanding contributions to clinical research and advocacy during its annual holiday and awards dinner Nov. 29 in Indianapolis.
Hoimes presents GU14-188 study at ESMO

Christopher J. Hoimes, DO, presented the Hoosier Cancer Research Network study GU14-188 at a poster discussion session October 20 during the 2018 European Society of Medical Oncology (ESMO) Congress in Munich, Germany. Dr. Hoimes is sponsor-investigator of the study of neoadjuvant pembrolizumab and chemotherapy for locally advanced urothelial cancer. See the HCRN GU14-188 poster and […]
HCRN staff present, receive recognition at Onsemble Conference
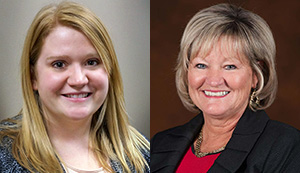
Hoosier Cancer Research Network (HCRN) Executive Director Cyndi Burkhardt, RN, will speak during the Onsemble Conference, September 18-21 in Madison, Wis., and Assistant Director for Clinical Systems Adelai Neal will be recognized with an award for her contributions to the Onsemble community. Onsemble is a semi-annual customer-exclusive conference hosted by Forte, a company that specializes […]