Hoosier Cancer Research Network is committed to improving the lives of cancer patients through clinical research. Study participants allow researchers to conduct studies that will improve outcomes for future generations of cancer patients.
Clinical trials
Research sites
Subjects
HCRN brings together researchers from across the United States to conduct collaborative investigator-initiated studies that address critical research questions across all cancer types.
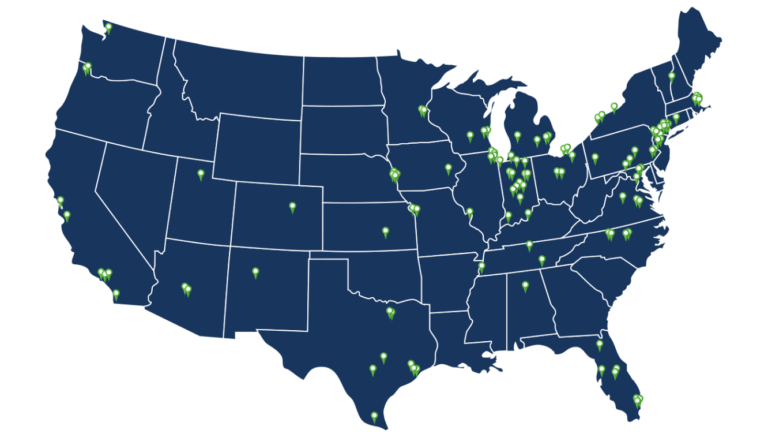
HCRN clinical trials are offered through our network of more than 100 academic and community sites throughout the United States. More than 500 researchers across cancer types participate in HCRN’s Clinical Trial Working Groups.
Sort by:
Sort by:
Current Trials
Our Network
Our Story