HCRN-LYM22-565 presented at EHA 2025 Congress

Dipenkumar Modi, MD, of the Karmanos Cancer Institute recently presented an interim analysis of the HCRN-LYM22-565 study at the European Hematology Association (EHA) 2025 Congress, held June 12–15 in Milan, Italy.
HCRN’s Report Reveals Key Trends Shaping Investigator-Initiated Oncology Trials
Hoosier Cancer Research Network has released a new report, “Investigator-Initiated Oncology Trials: Trends, Challenges, and Opportunities,” that captures the experience and perspectives of more than 130 researchers across its nationwide network. The findings highlight key trends shaping investigator-initiated oncology research and the operational, institutional, and funding challenges researchers face in today’s environment.
CEO Brian Stemme: A Leader in Life Sciences

We are proud to see our CEO, Brian Stemme, featured in an Indiana Business Journal article as a trusted expert in Indiana’s life sciences ecosystem.
HCRN’s presence at ASCO 2025
Hoosier Cancer Research Network (HCRN) is set to participate in the American Society of Clinical Oncology® (ASCO) 2025 Annual Meeting in Chicago from May 30 to June 3. During the event, four HCRN studies will be presented, working group meetings will take place, and HCRN will host a joint reception with the Big Ten Cancer […]
Julie Russell Dilts joins HCRN as General Counsel

Julie Russell Dilts, JD, has joined the Hoosier Cancer Research Network (HCRN) team as general counsel. Julie brings a wealth of expertise in various legal and regulatory areas, as well as a passion for the mission of HCRN.
HCRN launches new website

Hoosier Cancer Research Network (HCRN) launched an updated website today with new features and a modernized design.
Correlative analysis from HCRN GU16-260 study published in Cancer Discovery

Researchers participating in the Hoosier Cancer Research Network GU16-260 study have published a correlative analysis in Cancer Discovery. The analysis, led by David A. Braun, MD, PhD, of Yale Cancer Center; Catherine J. Wu, MD, of the Dana-Farber Cancer Institute; and Michael B. Atkins, MD, of Georgetown Lombardi Comprehensive Cancer Center, found that the presence of […]
Five HCRN studies presented at ASCO GU 2025, with HCRN-GU16-243 as a rapid oral abstract

Hoosier Cancer Research Network (HCRN) is set to participate in ASCO GU 2025 in San Francisco from February 13-15, 2025, with HCRN-GU16-243 being presented as a Rapid Oral Abstract, and several other studies as poster presentations.
HCRN-LUN21-497 presented at SITC 2024

A Hoosier Cancer Research Network abstract, HCRN-LUN21-497, titled, “Results from a phase II study of GT103 in combination with pembrolizumab in refractory, metastatic non-small cell lung cancer,” was presented at SITC 2024 by Jeffrey Clarke, MD, of Duke University.
HCRN-GI23-643 presented at ASCO GI 2025

HCRN-GI23-643, titled, “Randomized Trial of Regorafenib in Combination with Pembrolizumab or Pembrolizumab Monotherapy for Patients with MSI-H Colorectal Cancer,” will be presented as a Trials in Progress poster at the American Society of Clinical Oncology® (ASCO) Gastrointestinal Cancers Symposium 2025 in San Francisco. This study is led by Ibrahim Sahin, MD, of UPMC Hillman Cancer Center.
HCRN-GU16-243 presented at SUO 2024

A correlative study related to the Hoosier Cancer Research Network (HCRN) clinical trial, HCRN-GU16-243, titled, “A Phase 1 Trial of Durvalumab in Combination with Bacillus Calmette-Guerin (BCG) or External Beam Radiation Therapy in Patients with BCG-unresponsive Non-muscle-Invasive Bladder Cancer: The Hoosier Cancer Research Network GU16-243 ADAPT-BLADDER Study,” was presented at SUO 2024 by Noah Hahn, MD, […]
HCRN Non-Small Cell Lung Cancer Study Published in Journal of Clinical Oncology

A Hoosier Cancer Research Network (HCRN) study, HCRN-LUN18-335, led by Xiuning Le, MD, PhD, of the University of Texas MD Anderson Cancer Center, was published in the Journal of Clinical Oncology on October 8, 2024. The article is titled, “A Multicenter Open-Label Randomized Phase II Study of Osimertinib With and Without Ramucirumab in Tyrosine Kinase […]
HCRN-LUN21-497 Presented at SITC 2024 Annual Meeting

HCRN investigators presented results from the HCRN-LUN21-497 study as a poster at the Society for Immunotherapy of Cancer (SITC) 2024 Annual Meeting, held November 8–10, 2024, in San Diego, California.
Rita Assi, MD, Elected President-Elect of Indiana Oncology Society 2024-2026 Board of Directors

Hoosier Cancer Research Network congratulates Rita Assi, MD, on her election as the President-Elect of the Indiana Oncology Society 2024-2026 Board of Directors. Dr. Assi is a board member of HCRN and is assistant professor of clinical medicine at the Indiana University Melvin and Bren Simon Comprehensive Cancer Center.
40 Years of friendship and collaboration: A conversation with HCRN co-founders Pat Loehrer and Bill Fisher
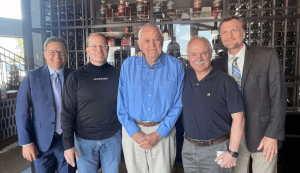
Hoosier Cancer Research Network (HCRN) recently joined two of our visionary founders, Patrick J. Loehrer, MD, and William B. Fisher, MD, as they shared stories spanning their 40 years of involvement with HCRN, formerly known as Hoosier Oncology Group, or affectionately, the “HOG.”
HCRN-LUN18-357 accepted to World Conference on Lung Cancer

A Hoosier Cancer Research Network trial, HCRN-LUN18-357, titled “P1.08D.03 Induction Durvalumab Followed by Chemoradiation and Consolidation Durvalumab for Stage III Non-Small Cell Lung Cancer (NSCLC)” has been accepted as a poster presentation and will be presented at the World Conference on Lung Cancer Meeting on September 8, 2024 from 12:00 pm-2:00 pm.
HCRN 2023-24 Manuscripts & Meeting Abstracts

Hoosier Cancer Research Network is happy to celebrate the research accomplishments of our members over the past year. Congratulations to all researchers and study teams whose hard work produced 23 journal manuscripts and meeting abstracts between July 1, 2023, and June 30, 2024!
HCRN-GU22-587 presented at Kidney Cancer Research Summit

A Hoosier Cancer Research Network trial, HCRN-GU22-587, titled, “Advanced Renel Cell Cancer Combination Immunotherapy Clinical Trial,” was presented this week at the Kidney Cancer Research Summit (KCRS). Michael Serzan, MD (pictured right), of Dana-Farber Cancer Institute, presented the study as a trial in progress.
Hoosier Cancer Research Network Article Published in JSCDM

Hoosier Cancer Research Network (HCRN) is proud to share that an article written by former HCRN team members, Tim Breen and Adelai Neal, titled, “The Endpoints Dataset: A Quality Control Method for Review and Analysis of Critical Efficacy Endpoints Data,” has been published in the Spring Issue 2024 of the Journal of the Society for […]
HCRN CEO Brian Stemme Featured on Purdue Pathfinder Podcast

Brian Stemme, Hoosier Cancer Research Network’s (HCRN) chief executive officer, was recently featured on Pathfinder, a Purdue Life Sciences podcast with Dr. Thomas G. Sors from Purdue Institute of Inflammation, Immunology and Infectious Disease (PI4D). Stemme shared insights on his career journey, why Indiana is a top spot for life sciences and HCRN’s impactful work.
Former HCRN leader, Dr. Robin Zon, appointed ASCO president

Hoosier Cancer Research Network congratulates Dr. Robin Zon on assuming her new role as president of the American Society of Clinical Oncology. Dr. Zon served as HCRN’s vice chair from 2006-2009, and as chief community officer from 2009-2010.
Belief-driven workforce: Insights from HCRN’s Brian Stemme

Chief Executive Officer of Hoosier Cancer Research Network, Brian Stemme, authored an article on Inside Indiana Business titled, “Belief-driven employees seek organizations that make a difference,” published May 24, 2024.
HCRN’s Presence at ASCO 2024

Hoosier Cancer Research Network (HCRN) is set to participate in the American Society of Clinical Oncology® (ASCO) 2024 Annual Meeting in Chicago from May 31 to June 4. During the event, five HCRN studies will be presented, working group meetings will take place, and HCRN will host a joint reception with the Big Ten Cancer […]
Reps for Research Update

Hoosier Cancer Research Network is excited to share that Reps for Research 2024 has raised $3,060, bringing our 7-year cumulative total to more than $26,000. Reps for Research took place on March 3, 2024, as fundraiser for Hoosier Cancer Research Network (HCRN) led by Christopher A. Fausel, chairman of HCRN’s board of directors and oncology […]
HCRN lymphoma working group co-chairs driven by science and mentorship

Co-chairs of the HCRN Lymphoma Working Group, Jonathan B. Cohen, MD, Natalie Galanina, MD, and Natalie Grover, MD, were driven by science and the influence of strong mentors early in their careers. It’s also why lymphoma is a focus of their work as they see great opportunities to make an impact through the WG collaboration. […]
Reps for Research is back

Hoosier Cancer Research Network is excited to announce that Reps for Research is returning March 3, 2024, after a three-year hiatus.
HCRN study in Merkel cell now enrolling

A new Hoosier Cancer Research Network clinical trial studying Merkel cell carcinoma is currently recruiting subjects. The trial, A Single Arm Phase II Study with Safety Run-in of Peptide Receptor Radionuclide Therapy (PRRT) in Combination with Immunotherapy for Patients with Merkel Cell Cancer (HCRN-MCC20-443; iPRRT Study), is led by Pashtoon Kasi, MD, MS, of Cornell […]
HCRN sarcoma co-chairs united in vision and goals for new working group

2023 marked the establishment of a new Sarcoma Clinical Trial Working Group for HCRN. The group brings together some of the sharpest minds in cancer research who are focused on one of the rarest types of cancer. The working group is led by (pictured from left) Atrayee Basu-Mallick, MD, interim director, Solid Tumor Division, and […]
HCRN GI14-198 published findings in European Journal of Cancer

Researchers participating in HCRN GI14-198, recently reported findings in the European Journal of Cancer. The study was a phase II randomized, double-blind study of mFOLFIRINOX plus ramucirumab versus mFOLFIRINOX plus placebo in advanced pancreatic cancer patients.
HCRN study featured in trials in progress at SNO Annual Meeting

Researchers participating HCRN BRE21-516, also known as BRIDGET, had their work featured as a Trials in Progress abstract at the Society for NeuroOncology Annual Summit. The study focuses on secondary brain metastases prevention after isolated intracranial progression on trastuzumab/pertuzumab or T-DM1 in patients with advanced human epidermal growth factor receptor 2+ breast cancer with the addition of tucatinib.
HCRN lung cancer study published in The Oncologist

HCRN LUN13-175 recently had its findings published in The Oncologist. Led by Nisha Mohindra, MD of Northwestern, this trial was a phase I/II trial of carboplatin, Nab-paclitaxel, and pembrolizumab for advanced non-small cell lung cancer (NSCLC).
ASCO honors Patrick J. Loehrer, MD, with Humanitarian Award

During the 2023 ASCO Annual Meeting, Patrick J. Loehrer, MD, FACP, FASCO, received the ASCO Humanitarian Award in recognition of his work personifying ASCO’s mission and values as well as providing service and leadership both at home and abroad.
HCRN investigators present three abstracts during ASCO 2023

Three Hoosier Cancer Research Network (HCRN) investigator-initiated clinical trials were highlighted during the American Society of Clinical Oncology (ASCO) 2023 Annual Meeting in Chicago.
Meet Hoosier Cancer Research Network at ASCO 2023 Annual Meeting

Hoosier Cancer Research Network (HCRN) will have an exhibitors booth at the American Society of Clinical Oncology (ASCO) 2023 Annual Meeting. Visit Booth #28115 to talk with our highly qualified staff about our 35 years of experience managing oncology/hematology multi-center clinical trials.
HCRN Urothelial Cancer Study presents findings at 2023 ASCO GU Cancers Symposium

Hoosier Cancer Research Network urothelial cancer study was highlighted during the 2023 ASCO Genitourinary Cancers Symposium in San Francisco. In a poster and rapid abstract session presentation during the 2023 ASCO Genitourinary Cancers Symposium. Jason Brown, MD, PhD, of University Hospitals Seidman Cancer Center presented the results of HCRN GU14-188, a Phase Ib/II trial of […]
HCRN bladder cancer study published in European Urology

Researchers participating in the study known as ADAPT-BLADDER, or HCRN GU16-243, recently reported preliminary findings in the journal European Urology. The study evaluated the safety and preliminary efficacy of anti-PD-L1 directed therapy with durvalumab (cohort 1), durvalumab plus Bacillus Calmette-Guerin (BCG) (cohort 2) and durvalumab plus external beam radiation therapy (EBRT) (cohort 3).
HCRN Remembers Alesha Arnold, RN: A dedicated board member committed to finding a cure

It is with heavy hearts we share the passing of Hoosier Cancer Research Network Board Member Alesha Arnold, RN. Alesha was a breast oncology research nurse at the IU School of Medicine and supported the Clinical Trials Office at the IU Melvin and Bren Simon Cancer Center. Alesha worked with Clarian Health from 1997 to […]
Investigator Spotlight: Bently Doonan, MD, MS

Hoosier Cancer Research Network highlights Bently Doonan, MD, MS, oncologist and assistant professor in the division of hematology & oncology at the university of Florida College of Medicine.
Three HCRN studies featured during 2023 ASCO GU Cancers Symposium

Three Hoosier Cancer Research Network genitourinary studies were featured during the American Society of Clinical Oncology Genitourinary (GU) Cancers Symposium in San Francisco, CA on February 16-18, 2023.
HCRN investigators present three studies during 2023 ASCO Gastrointestinal Cancers Symposium

Three Hoosier Cancer Research Network (HCRN) investigator-initiator clinical trials will be highlighted during the American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium in San Francisco, CA.
HCRN seeks Communications Intern

Hoosier Cancer Research Network (HCRN) is currently seeking a part-time communications intern. Students working toward a college degree in Marketing/Communications, Advertising, Public Relations or English are preferred.
Investigator Spotlight: Russell Pachynski, MD

Hoosier Cancer Research Network highlights Russell Pachynski, MD, genitourinary medical oncologist and associate professor of Medicine, Division of Medical Oncology, at Washington University School of Medicine.
Hoosier Cancer Research Network appoints life sciences industry veteran Brian Stemme as Chief Executive Officer

Hoosier Cancer Research Network (HCRN) announced today that Brian Stemme has been appointed Chief Executive Officer. Stemme, widely regarded as a champion of Indiana’s life sciences industry, will succeed Executive Director Cyndi Burkhardt, RN, who announced her retirement earlier this year. Stemme will assume responsibilities on Monday, August 29.
Investigator Spotlight: Sarah Sammons, MD

Hoosier Cancer Research Network highlights Sarah Sammons, MD a medical oncologist at Duke Cancer Center.
Investigator Spotlight: Richard Hall, MD, MS

Hoosier Cancer Research Network highlights Richard Hall, MD, MS, a medical oncologist at University of Virginia Cancer Center. Dr. Hall is a member of the HCRN Thoracic Clinical Trial Working Group.
Meet Hatim Sabaawy, MD, PhD: Correlative Sciences Clinical Trial Working Group Co-Chair

Meet Hatim Sabaawy, MD, PhD Hatim Sabaawy, MD, PhD, of the University of Colorado Cancer Center is the Director of Personalized Medicine in the Division of Medical Oncology at the CU School of Medicine. Dr. Sabaawy is responsible for fostering translational research activities across the academic and clinical partner institutions of the CU Cancer Center, […]
Investigator Spotlight: Pashtoon Kasi, MD, MS

Hoosier Cancer Research Network highlights Pashtoon Kasi, MD, MS, a medical oncologist at Weil Cornell Medicine. Dr. Kasi is a member of the HCRN Gastrointestinal Clinical Trial Working Group.
HCRN investigators present four studies during ASCO 2022 Annual Meeting

Four Hoosier Cancer Research Network (HCRN) investigator-initiated clinical trials were highlighted during the American Society of Clinical Oncology (ASCO) 2022 Annual Meeting in Chicago, IL. HCRN investigators presented one poster and three trials in progress posters during the 2022 ASCO annual meeting.
HCRN internship program creates a community and environment for students to grow

Four years ago, I left home to pursue higher education. Today, I feel a similar sense of nostalgia as this is my final day as an Intern at Hoosier Cancer Research Network. HCRN was not only a key to my financial stability in college, but it became a home consisting of the warmth only family […]
A letter from Cyndi Burkhardt, RN

Dear Colleagues: I am writing to let you know my plans to retire as Executive Director of Hoosier Cancer Research Network (HCRN) this year, once a successor is chosen and acclimated to the position. An executive search is currently underway. 8/1/22 update: Watch for an announcement soon introducing HCRN’s new executive director. I joined this […]

